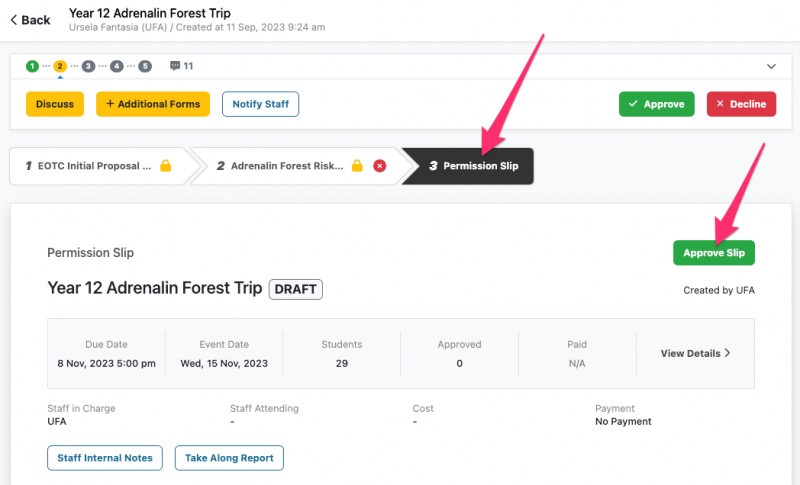
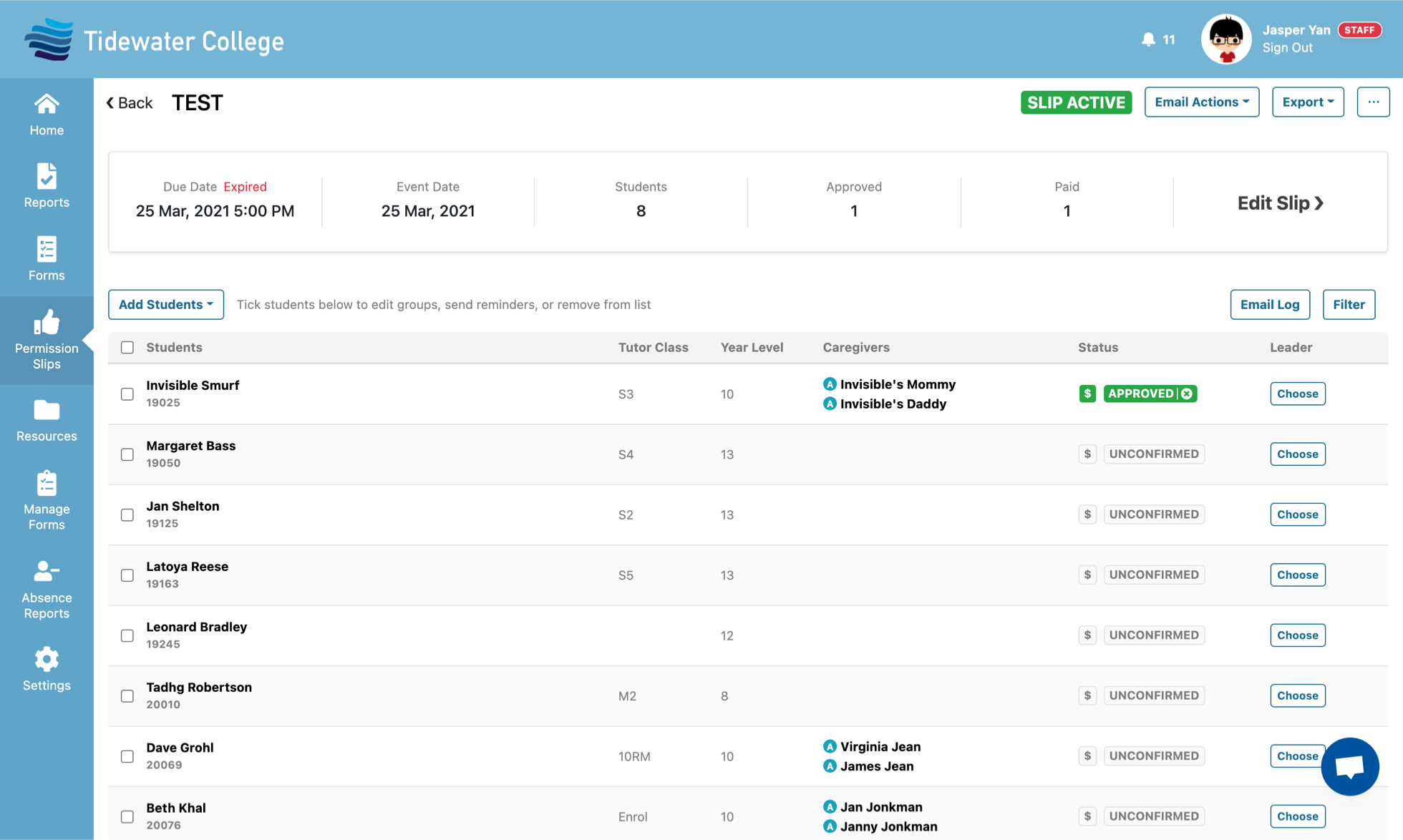
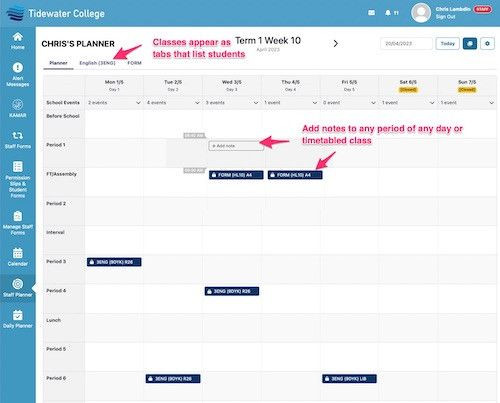
SchoolBridge is a collection of tools that help connect your school’s communities by leveraging your SMS data to help students, caregivers and staff share information and meet administrative obligations with less time and effort.
Meet Inbox Design - our Mount Maunganui based team of School Solution experts!
We create a range of specialised bespoke programs, aimed at New Zealand Schools to help your community of Learners, and your Staff.